
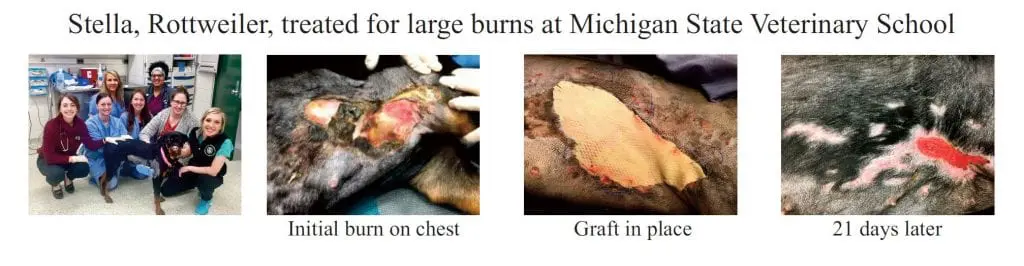
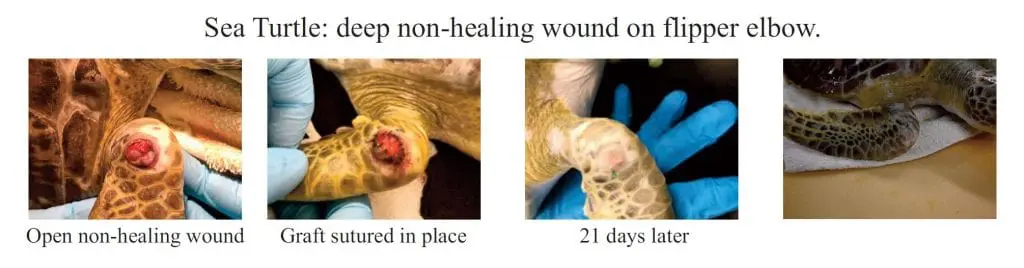
Kerecis VET is intact fish skin especially developed to manage damaged tissue such as partial and full thickness surgical wounds, trauma wounds, and burn wounds in small and large animals including mammals and exotic species.
The fish-skin grafts contain fat, protein, elastin, glycans and other natural skin elements and are supplied in multiple shapes, variants and sizes.
| wdt_ID | wdt_created_by | wdt_created_at | wdt_last_edited_by | wdt_last_edited_at | SKU | Product Description | Product Sizing | Total Units |
|---|---|---|---|---|---|---|---|---|
| 1 | avista | 27/02/2024 22:00 | hagustsson | 20/01/2025 13:29 | 80200S01B0D | Kerecis VET 3×3.5 cm | 3×3.5 cm | 10.5 cm² |
| 2 | avista | 27/02/2024 22:01 | avista | 17/01/2025 15:35 | 80200S02B0D | Kerecis VET 3×7 cm | 3×7 cm | 21 cm² |
| 3 | hagustsson | 17/01/2025 14:45 | avista | 17/01/2025 15:35 | 80200S04B0D | Kerecis VET 7×7 cm | 7×7 cm | 49 cm² |
| 4 | hagustsson | 17/01/2025 14:45 | avista | 17/01/2025 15:35 | 80200S03B0D | Kerecis VET 7×10 cm | 7×10 cm | 70 cm² |
| 5 | hagustsson | 17/01/2025 14:46 | avista | 17/01/2025 15:35 | 80200S21B0D | Kerecis VET 7×20 cm | 7×20 cm | 140 cm² |
| wdt_ID | wdt_created_by | wdt_created_at | wdt_last_edited_by | wdt_last_edited_at | SKU | Product Description | Product Sizing | Total Units |
|---|---|---|---|---|---|---|---|---|
| 1 | avista | 27/02/2024 22:00 | avista | 17/01/2025 15:35 | 80200P02D0D | VET Micro | 19 cm² | 19 cm² |
| 2 | hagustsson | 17/01/2025 14:50 | avista | 17/01/2025 15:35 | 80200P04D0D | VET Micro | 38 cm² | 38 cm² |
| 3 | hagustsson | 17/01/2025 14:50 | avista | 17/01/2025 15:35 | 80200P32D0D | VET Micro | 114 cm² | 114 cm² |
| wdt_ID | wdt_created_by | wdt_created_at | wdt_last_edited_by | wdt_last_edited_at | SKU | Product Description | Product Sizing | Total Units |
|---|---|---|---|---|---|---|---|---|
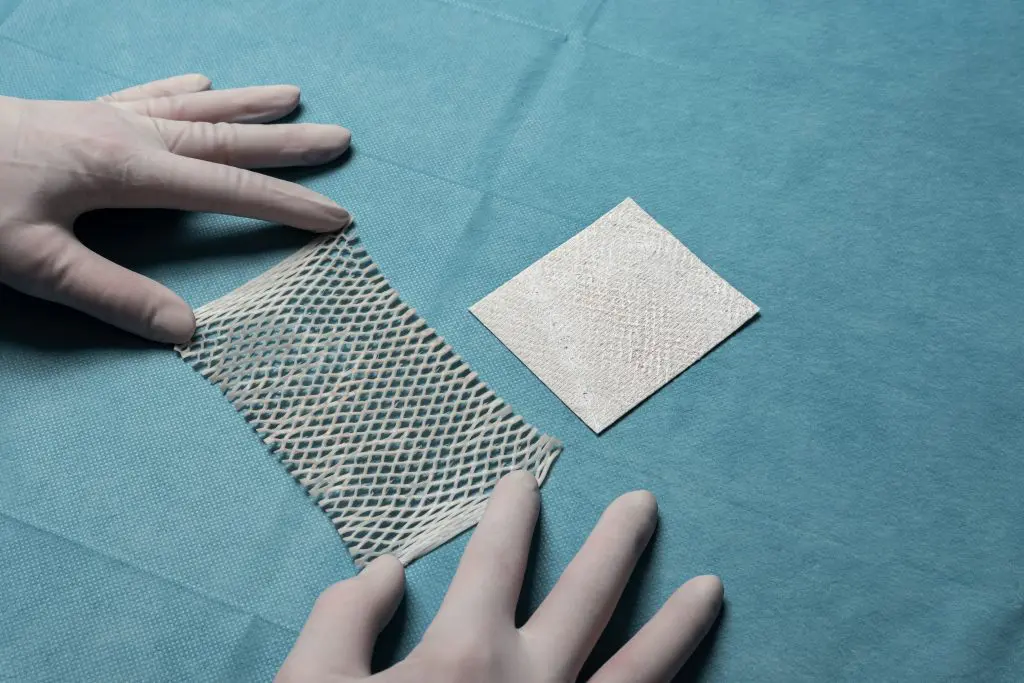
| 1 | avista | 27/02/2024 22:00 | hagustsson | 20/01/2025 13:30 | 80200N21B0D | Kerecis VET Meshed 2:1 | 7×20 cm | Up to 252 cm² |

This Site is not intended to provide diagnosis, treatment or medical advice. Products, services, information and other content provided on this Site, including information that may be provided on this Site directly or by linking to third-party websites, are provided for informational purposes only. Information on this Site should not be considered as a substitute for advice from a healthcare professional. Please consult with a physician or other healthcare professional regarding any medical or health related diagnosis or treatment options.
Products are not available in all countries and may be known by different names than used on this web-page. Products, product names, indications and claims vary between countries. Always make sure to check with your local health care professional and read the actual printed package information prior to use. Information on this website might not be current and might not be an accurate reflection of current product labeling.
A surgeon must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Kerecis does not dispense medical advice and recommends that surgeons be trained in the use of any particular product before using it in surgery.
The information presented is intended to demonstrate the Kerecis product offerings. A surgeon must always refer to the package insert, product label and/or instructions for use before using any Kerecis product. Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your Kerecis representative if you have questions about the availability of Kerecis products in your area.
KM-24-0043
