Groundbreaking study fills critical gap in evidence-based treatment options for DFUs with exposed structure.
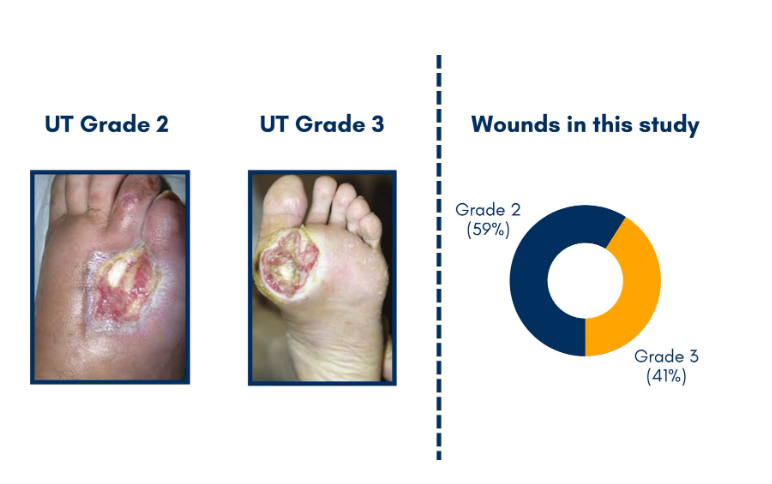
DFUs classified as University of Texas (UT) grades 2 and 3 are some of the most challenging wounds to treat, and are associated with higher rates of infection, delayed healing, and increased risk of amputation.1
The increased risk associated with grade 2 and 3 diabetic foot ulcers stems from their depth and complexity.
Increased Depth and Complexity creates:
- Penetration down through the dermis and subcutaneous tissue
- Depth creates environment for bacterial growth
- Exposed structures avascular and with limited capability for fighting infection
- Granulation tissue needs to migrate over exposed structures
For patients with University of Texas (UT) grade 2 and 3 ulcers – the severe cases where bone or tendon is exposed – the stakes of having an open wound are high:
- Higher risk of infection2
- Up to 80% increased risk of amputation3
- Increased healthcare costs per patient4
- 5-year mortality rates of up to 45%5
These complex ulcers have long been a challenge for clinicians, with limited evidence-based treatment options available. Despite the devastating impact these deep ulcers often have on patients and their families, there has been a lack of large-scale, randomized controlled trials to investigate care options.
Wound Closed at 16 Weeks
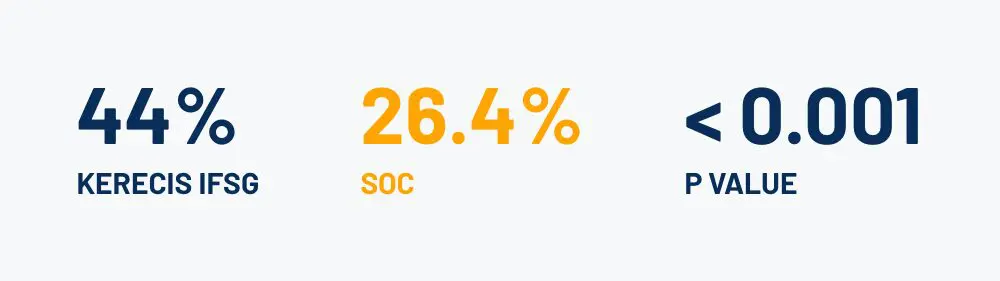
Significant Results: (95% CI: 1.48 – 4.56)