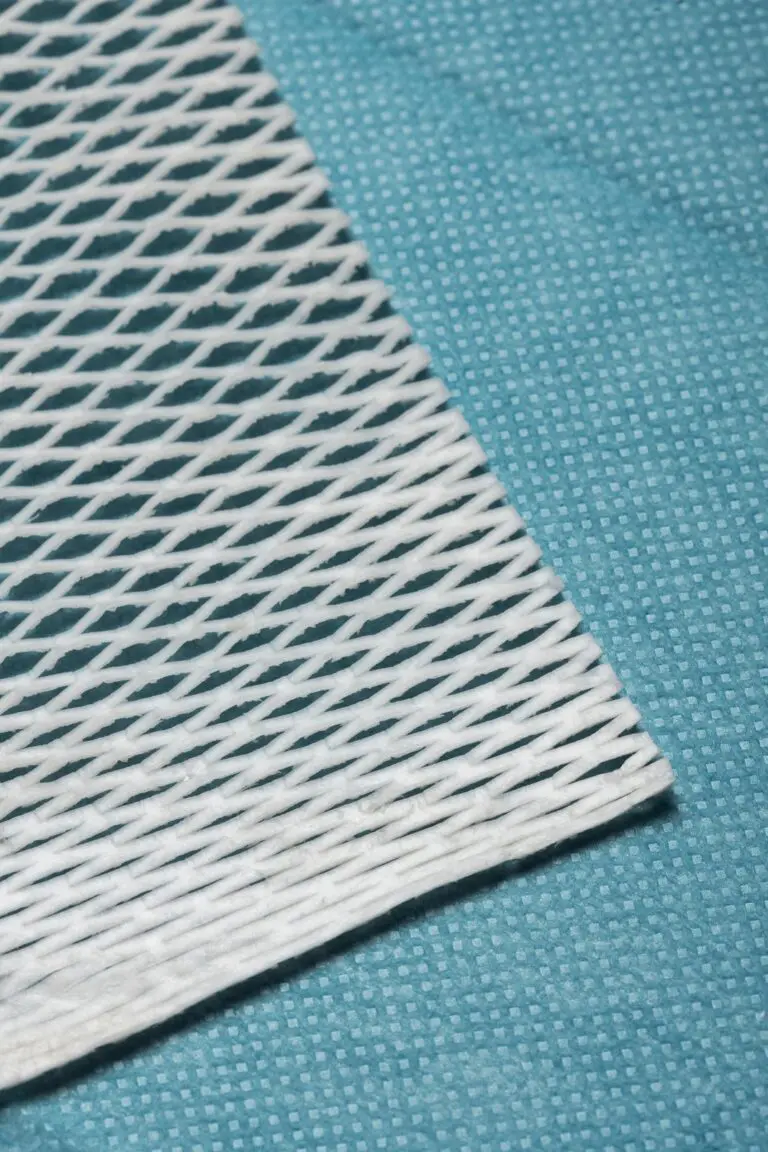
from North-Atlantic cod to humans, the fish skin only needs mild processing with our proprietary method.[2]
This process preserves the skin’s natural qualities[2], including: its three-dimensional structure, mechanical properties, molecular organization, and composition (including omega-3 fatty acids, glycosaminoglycans, proteoglycans, elastin, soluble collagens, and fibronectin).[1-4]
GraftGuide promotes healing with minimal impairment of functionality and positive cosmetic outcomes.[4] The product is homologous to human skin[1] and when applied
to damaged tissue, such as a burns or wounds, helps to support the body’s own cells to regenerate tissue.[5-12]