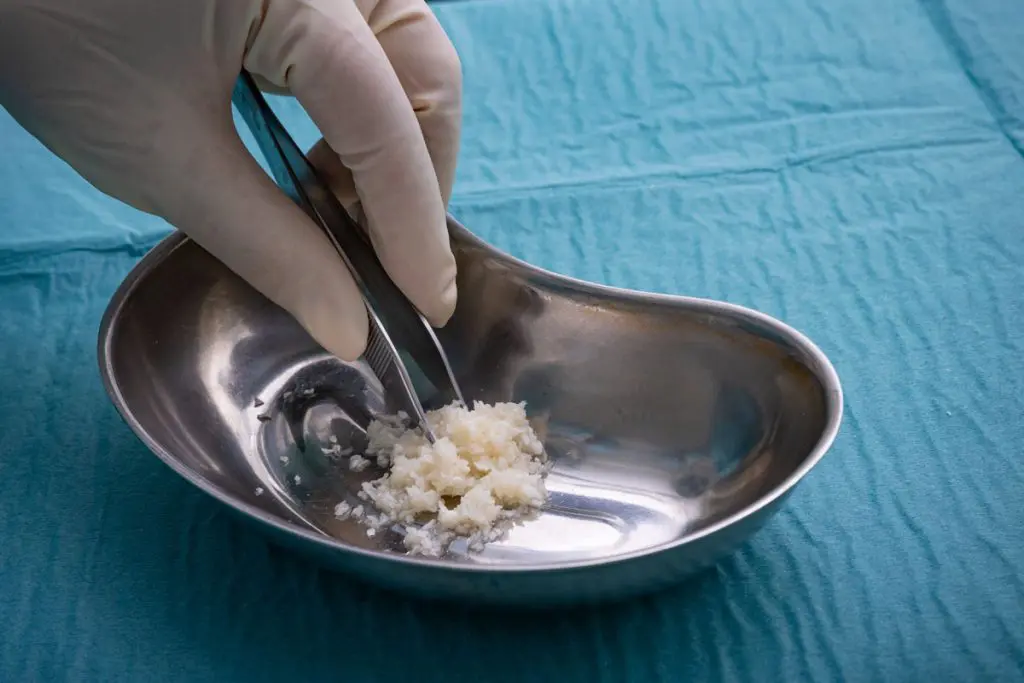
The easy-to-use fish-skin graft fills a void as wound-treatment companies withdraw human-derived products per FDA guidance
Arlington, VA and Reykjavik, Iceland – June 2, 2021 – Kerecis, the company pioneering the use of fish skin and fatty acids for tissue regeneration and protection, today announced the release of MariGen Micro™. The easy-to-use product is intact fish skin divided into fragments, which mold into wound beds. MariGen Micro is appropriate for treating complex trauma and uneven wounds in operating rooms, wound centers and doctors’ offices.
In recent years, several wound-treatment companies have marketed human-derived wound-treatment products under the FDA 361 pathway. Some of those products were fragmented or processed in other ways. The FDA has stipulated that, as of May 31, 2021, human-derived products that are “more than minimally manipulated” require a drug approval. In response, many companies have taken these products off the market rather than apply for such approval. This has reduced the number of products available to treat serious wounds. Kerecis is addressing this void with the introduction of MariGen Micro.
The easy-to-use graft is divided into small units that fill hard-to-reach areas, increasing the product’s effectiveness and improving healing. With a fragment size of about 0.15 cm, MariGen Micro can easily be applied in deep wound spaces that are not accessible to meshed or unmeshed sheets.
The product is approved for a range of wound conditions, including partial and full-thickness wounds; surgical and trauma wounds; and pressure, venous and diabetic ulcers.
“I found this product extremely easy to use,” said Ian Barron, DPM, of Clintonville/Dublin Foot & Ankle Group in Columbus, Ohio. “More importantly, I have seen even serious wounds heal quickly, some within days, without secondary procedures. The result is fewer procedures and an enhanced quality of life for our patients.”
“I am ecstatic to see the next evolution in the Kerecis product line,” said Patrick McEneaney, DPM, of the Northern Illinois Foot & Ankle Specialists in Chicago. “This product is extremely easy to use. The versatility of the Kerecis MicroGraft allows for optimal coverage of the most complex of tissue defects. I am amazed at the ability for this product to assist in tissue generation where traditional cellular tissue products have been difficult to utilize. I have seen faster healing times with less procedures leading to a sooner return to normal for my patients.”
Educational Presentation
To show how practitioners can achieve superior results using MariGen Micro, the company is having an educational presentation on “MariGen Micro Fish Skin in the Treatment of Complex Foot and Ankle Ulcerations.” Dr. Barron will present detailed cases where he has used the product in the treatment of complex foot and ankle ulcerations. John C. Lantis II, M.D., of Mount Sinai Hospital in New York, will moderate the session; and Kerecis founder and CEO Fertram Sigurjonsson will give opening remarks. The webinar will be June 3, 2021, at 7 p.m. ET. Registration is available here.
From the town of Ísafjörður in northwest Iceland, Kerecis develops, manufactures, and distributes patented fish-skin medical devices that support soft tissue regeneration in the body, with regulatory approvals in the United States, Europe, and beyond.