Kerecis welcomes the new draft Local Coverage Determination (LCD) policy recently proposed by seven Medicare Administrative Contractors (MACs), requiring manufacturers to document the clinical efficacy of their skin substitute products in the out-patient setting. Manufacturers of skin substitute products had an opportunity to provide their input to the draft policy during a commenting period that ended on June 8th, 2024. Kerecis submitted a comprehensive data-packet to the MACs that included; detailed information on the published randomized control studies that document the clinical efficacy of Kerecis products, reports from the analysis firms Avalere Health and Washington Analysis, as well as other data. The information in the data-package provide strong evidence to support the inclusion of the Kerecis MariGen and Kerecis Shield fish-skin grafts in the final policy and Kerecis strongly believes that the products will be covered in the final version of the policy.
Kerecis products in focus
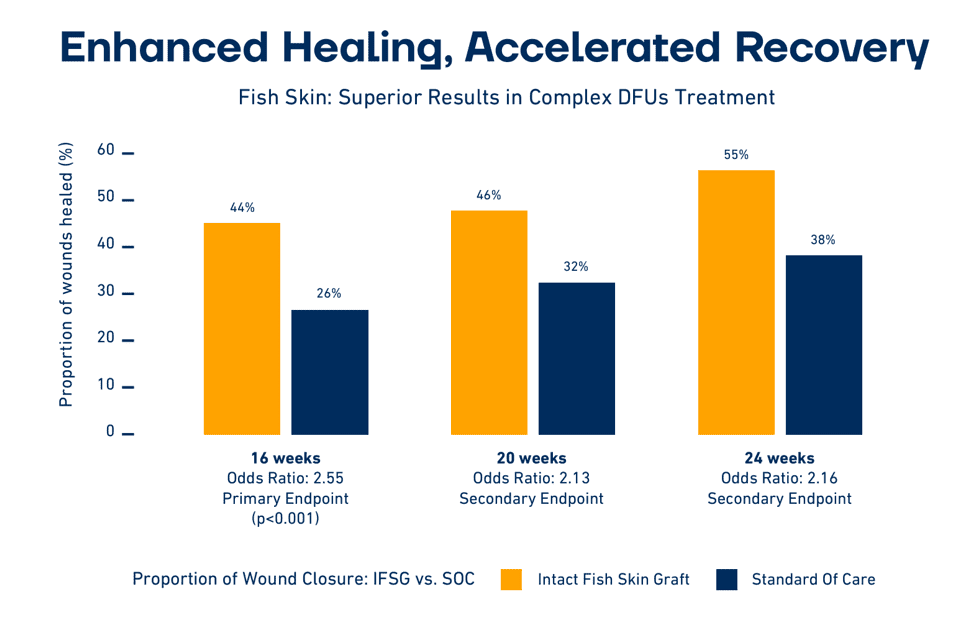
The draft LCD policy on Skin Substitute Grafts/Cellular and Tissue-Based Products for the Treatment of Diabetic Foot Ulcers and Venous Leg Ulcers that the MAC’s published on April 25, 2024 contains a clear technical qualification and clear clinical efficacy qualification that skin substitutes need to fulfill to be covered for payment. The draft policy also contains a list of products that are considered to meet both qualifications. Kerecis fulfils the technical qualification in the draft policy, but it is not named in the initial draft list of qualifying products. According to information received from the MACs this is because the draft product list, in the draft policy, was written before the publishing date of the Lantis 2023 study, which specifically addresses the efficacy of fish-skin in the management of chronic wounds. Since publishing, the Lantis 2023 study has resulted in over 100 million additional lives being covered by commercial payors in the US. Kerecis strongly believes that Kerecis product fulfill the clinical qualification and believes that Kerecis products will be on the list of covered products should the policy be implemented.
The MACs will now review the comments received during the commenting period and make changes to the policy as they see necessary. The MAC’s have until April 2025 to finalize the policy, or the draft policy will automatically retire. The MACs must publish a final policy at least 45 days before the effective date. Kerecis anticipates that the earliest the draft policy will become effective is the final quarter of 2024, but per the above it could take until April 2025.
Until the final LCD out-patient policy is posted and becomes effective, Kerecis products will continue to be covered for the treatment of diabetic foot ulcers and venous leg ulcers in the out-patient setting. We do not anticipate any disruption to coverage or business in the out-patient setting in the coming months.
Key Kerecis points:
Kerecis is confident that upon review of the comprehensive data-package submitted, the MACs will include the Kerecis MariGen and Kerecis Shield products in the final policy, ensuring continued access to these innovative and effective wound care solutions for Medicare beneficiaries.
To access the full comment letter and supporting documents, click the links below.
For any questions or further information, contact Dr. Gunnar Johannsson, SVP & Medical Director, gj@kerecis.com.
Key studies: