Bremen Germany — May 5, 2016 — Kerecis, the company using Omega3-rich fish skin to heal human wounds and tissue damage, will present results of human and laboratory trials at the European Wound Management Symposium (EWMA) May 11-13 at the Bremen Messe in Germany. Kerecis is exhibiting in Booth 1A 05.
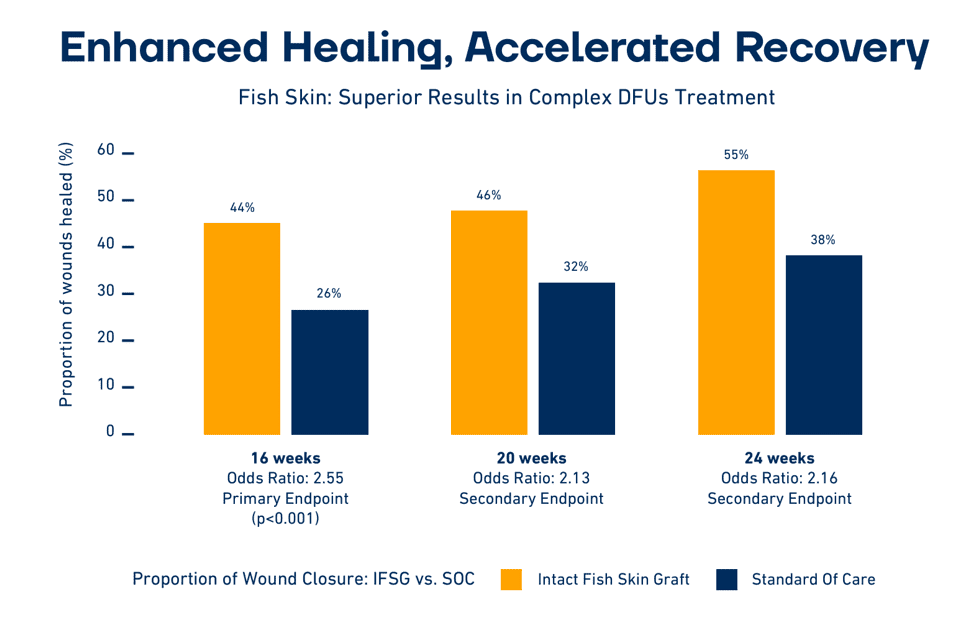
In one trial, the fish-skin treatment resulted in a 39 percent reduction in the use of antibiotics. Another trial showed superior cell ingrowth using the fish-skin treatment compared to the leading human-amnion-membrane products used in wound treatment.
The problem of chronic wounds continues to grow globally with the increase in diabetics, obesity and age. It is estimated that 25 to 50 percent of acute hospital beds in Europe are occupied by patients with at least one wound. Germany has four million chronic wounds per year; 30 000 of them require amputation, depriving many people of mobility. The average cost of a leg wound in Europe is EUR 6.650 and accounts for 2 to 4 percent of the total health care budget. It’s logical that these costs will increase significantly in the coming years if no action is taken.
About 6,000 medical professionals attend the annual EWMA conference, which will feature more than 1,000 scientific presentations on wound treatment and amputation prevention.
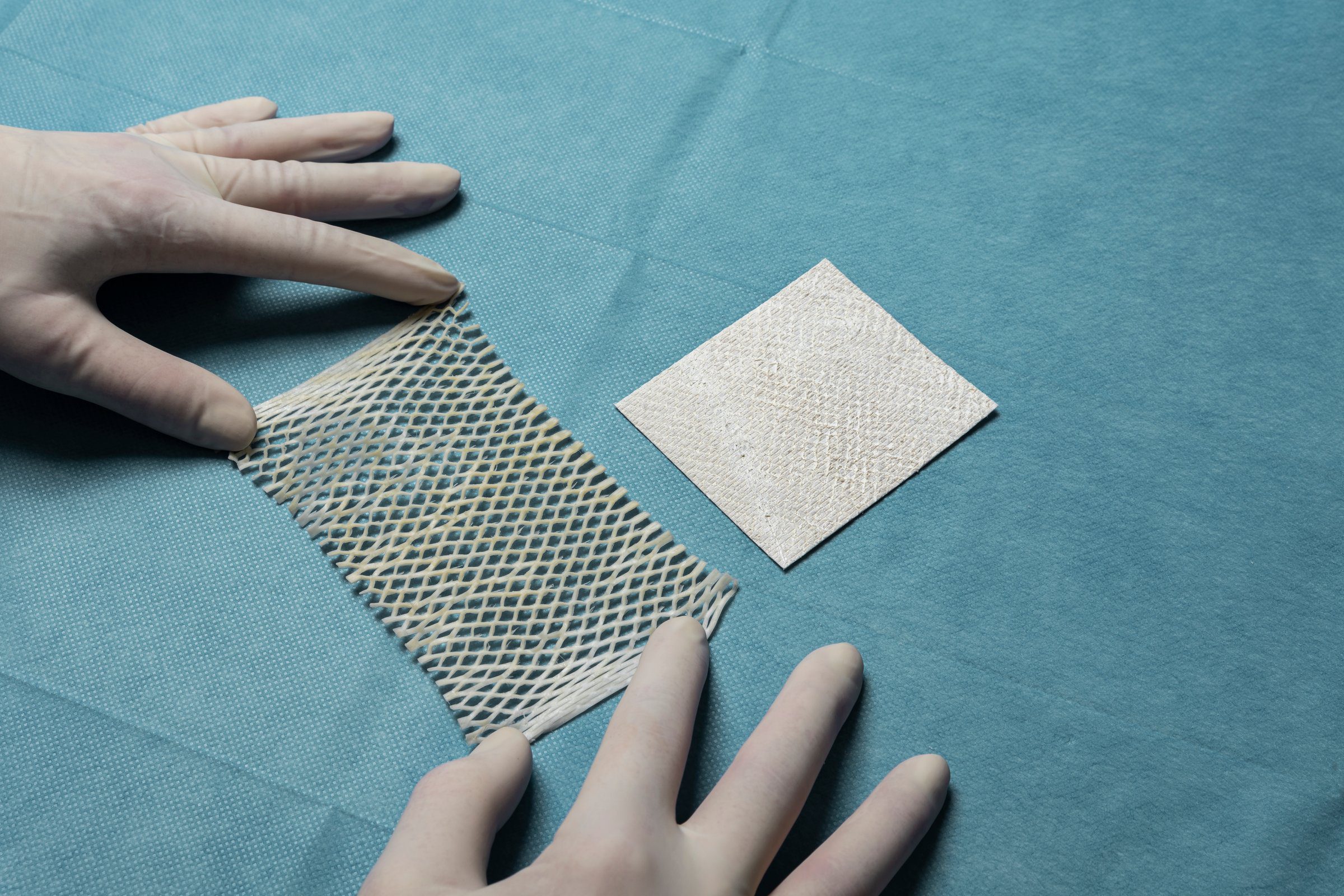
Kerecis Omega3 is intact fish skin that is rich in naturally occurring Omega3 polyunsaturated fatty acids and is used to regenerate damaged human tissue. When grafted onto damaged human tissue, such as a diabetic ulcer, the acellular material recruits the body’s cells from the wound perimeter. These cells are then incorporated into the fish skin, which is ultimately converted into functional, living tissue. The fish skin structure resembles the native structure of human skin. Studies have shown that cells and stem cells proliferate faster in this structure than in other materials such amnion-membrane and other mammalian-sourced materials. The Kerecis product is available in several European countries.
Wounds in Europe are largely treated with second-generation wound-treatment products such as polyurethane foam and hydrocolloids. “We intend to lead the transition to third-generation biologic products, which have been routinely used in the U.S. for years, and are now making their inroads into Europe,” said Fertram Sigurjonsson, founder and CEO of Kerecis. “Our scientific results show that our technology can help improve care and reduce amputations. We will present the results of seven trials and tests at the conference that strengthen the scientific positioning of our product and help bring this new technology into mainstream use, potentially reducing the number of amputations,” he added.
During EWMA, Dr. John Lantis of Mount Sinai Hospital (New York City) will present the following trial results:
The following poster abstracts, which contain trial results for the Kerecis Omega3 technology, will also be presented on the conference: