Millions more Americans are now eligible for insurance coverage for Kerecis’ medical-fish-skin technology. Many U.S. private insurers recently rated Kerecis Omega3 MariGen as “medically necessary” for diabetic foot ulcers that have not healed with standard therapy. (Photo: Business Wire)

REYKJAVIK, Iceland & ARLINGTON, Va.–(BUSINESS WIRE)–Kerecis announced today that, over the past year, 63 million more Americans became eligible for insurance coverage for the company’s fish-skin treatments, an increase of more than 70%. This is the result of more private insurance companies deciding to cover the Kerecis fish-skin technology, in addition to previously existing Medicare coverage. This milestone means that about 150 million Americans — about 45% of the country’s population — can now enjoy the benefits of the patented fish-skin treatment.
“A significant portion of the population now has access to our fish-skin treatment. We expect that the number of people covered by insurance for our products will increase from 150 million today to more than 200 million in the coming year”
Kerecis is pioneering the use of sustainably sourced fish skin and fatty acids in cellular therapy and tissue regeneration and protection. The company has three product lines indicated for the treatment of damaged human tissue: GraftGuide® for burns, SurgiBind/SurgiClose® for surgical repair, and MariGen for conditions such as diabetic wounds, pressure ulcers and vascular ulcers.
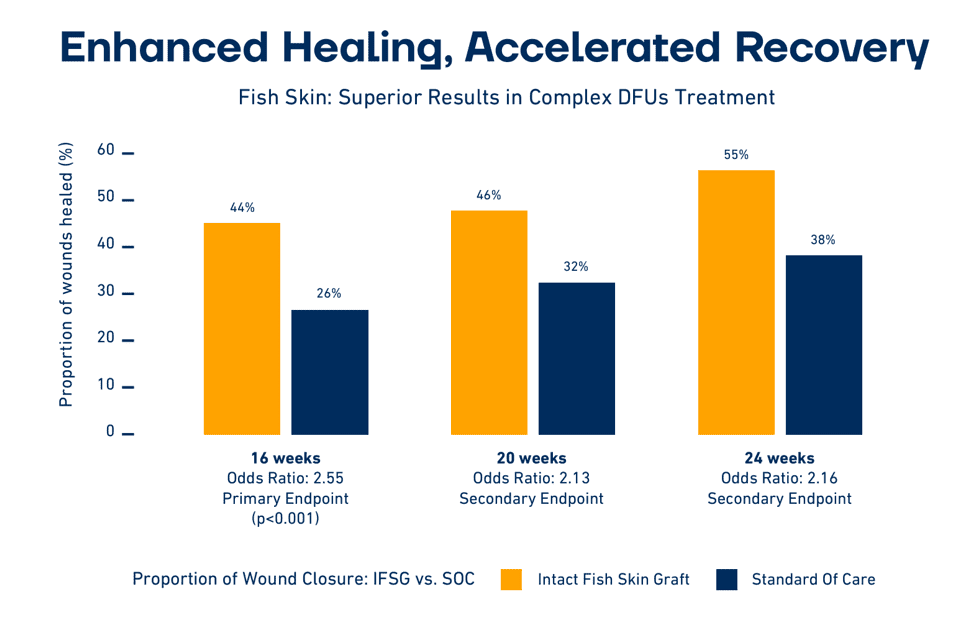
The increased coverage is partially due to the fact that multiple large U.S. private insurers recently rated MariGen as “medically necessary” for diabetic foot ulcers that have not healed with standard therapy.
“This substantial growth in insurance coverage reflects the increased recognition of our treatment’s efficacy and economy of use,” said Fertram Sigurjonsson, founder and CEO of Kerecis. “A significant portion of the population now has access to our fish-skin treatment. We expect that the number of people covered by insurance for our products will increase from 150 million today to more than 200 million in the coming year,” he added.
The Kerecis technology has been the subject of more than 50 peer-reviewed studies including four randomized, controlled trials. Many of these studies have found that the Kerecis products heal wounds faster than alternative products.